Understanding the Ionization Energy Trend: What You Need to Know
Table of Contents
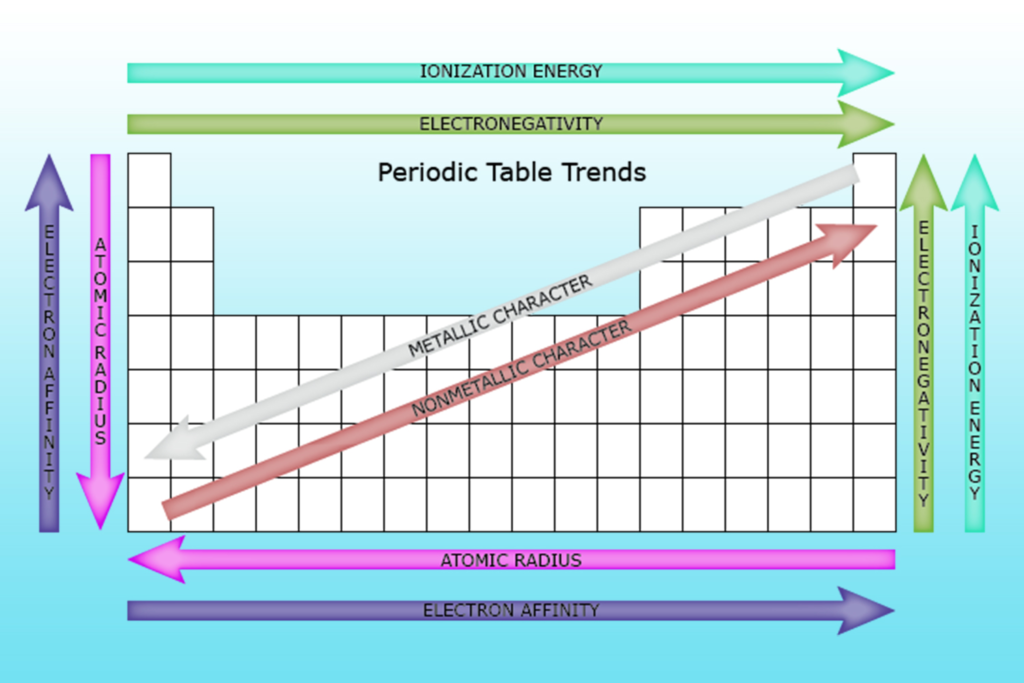
The ionization energy trend refers to the pattern of how the energy required to remove an electron from an atom changes as you move across the periodic table. This trend helps us understand how atoms behave and how strongly their electrons are held by the nucleus. In this blog, we will explore the ionization energy trend, its importance, and how it can change in different parts of the periodic table.
In general, the ionization energy trend shows that as you move from left to right across a period, the energy needed to remove an electron increases. This happens because atoms become smaller, and the electrons are pulled closer to the nucleus. On the other hand, as you move down a group in the periodic table, the ionization energy decreases. This is due to the increased distance between the outer electrons and the nucleus, making it easier for the atom to lose an electron.
What Is the Ionization Energy Trend and Why Does It Matter
The ionization energy trend helps us understand how much energy is needed to remove an electron from an atom. This trend shows how this energy changes when we move across the periodic table. By studying this trend, scientists can learn how elements behave and why some are more likely to form positive ions than others.
The ionization energy is important because it explains why certain elements are reactive while others are stable. For example, metals like sodium lose electrons easily, while non-metals like oxygen hold onto their electrons tightly. This difference is based on the ionization energy trend in the periodic table.
How Ionization Energy Trend Changes Across the Periodic Table
As we move from left to right across a period in the periodic table, the ionization energy increases. This happens because the atoms get smaller, and the electrons are pulled closer to the nucleus.
- Smaller atoms: Atoms become smaller as we go across a period. This means the outer electrons are closer to the nucleus, so they are harder to remove.
- Increased nuclear charge: As the number of protons in the nucleus increases, the pull on the electrons becomes stronger.
On the other hand, as you go down a group in the periodic table, the ionization energy decreases. This is because the outer electrons are farther away from the nucleus, making it easier to remove them.
The Role of Nuclear Charge in the Ionization Energy Trend
The nuclear charge plays a big role in the ionization energy trend. Nuclear charge refers to the total charge in an atom’s nucleus, which is determined by the number of protons.
- More protons: A higher nuclear charge increases the attraction between the nucleus and electrons, raising the ionization energy.
- Less shielding: As more protons are added, the electrons in the same shell experience less shielding from the nucleus. This makes it harder for outer electrons to escape.
When you move across a period from left to right, the nuclear charge increases. This is why the ionization energy increases across a period—because the electrons feel a stronger pull from the nucleus.
Why Does Ionization Energy Decrease Down a Group
Ionization energy decreases as you move down a group in the periodic table. The main reason for this is the increasing number of electron shells.
- More electron shells: Each new shell adds more distance between the nucleus and the outermost electron. This makes it easier to remove the electron, lowering ionization energy.
- Increased shielding: As the number of inner electrons increases, they shield the outermost electrons from the pull of the nucleus. This reduces the effective nuclear charge felt by the outer electrons.
Because of these factors, it requires less energy to remove an electron from an atom lower in a group compared to one higher up in the same group.
Factors That Influence the Ionization Energy Trend
Several factors influence the ionization energy trend in the periodic table. These include atomic size, electron shielding, and nuclear charge. Understanding these factors helps explain why the ionization energy trend behaves in certain ways.
- Atomic size: Smaller atoms have higher ionization energies because their electrons are closer to the nucleus, making them harder to remove.
- Electron shielding: More electron shells create more shielding, which reduces the nuclear pull on outer electrons and lowers ionization energy.
- Nuclear charge: A higher nuclear charge increases ionization energy because it strengthens the attraction between the nucleus and the electrons.
Conclusion
In conclusion, the ionization energy trend is an important concept that helps us understand how atoms interact and behave. It shows how the energy needed to remove an electron from an atom changes across the periodic table.
The trend also helps us understand the differences between elements in a group and a period. As we move across a period, ionization energy increases, while it decreases as we move down a group.
FAQs
Q: What is ionization energy?
A: Ionization energy is the energy needed to remove an electron from an atom. It helps us understand how strongly an atom holds onto its electrons.
Q: Why does ionization energy increase across a period?
A: Ionization energy increases across a period because atoms get smaller, and the nucleus pulls electrons closer, making them harder to remove.
Q: Why does ionization energy decrease down a group?
A: Ionization energy decreases down a group because the atoms get larger, and the outer electrons are farther from the nucleus, making them easier to remove.
Q: What is the shielding effect?
A: The shielding effect occurs when inner electrons block the pull of the nucleus on outer electrons, making it easier to remove them.
Q: How does nuclear charge affect ionization energy?
A: A higher nuclear charge makes ionization energy higher because the stronger pull from the nucleus makes it harder to remove an electron.